IRB Submissions
IRB Protocol submissions are accomplished through a cloud-based system.
Note: The system uses your UAkron credentials. When you first sign in, you are NOT in the Human Ethics module.
Navigate to Human Ethics by choosing "Products" (in the upper right of the window) and then "Human Ethics."
HRPP video trainings
Links to screenshot trainings put together by Cayuse: Researchers: Creating and Managing Studies and Submissions
Cayuse HE FAQs
Registrations
- Registrations will not be input into Cayuse HE, as no additional follow up is required for these categories.
- Remember, changes that cause the project to become research must be submitted to the IRB prior to implementation. A research protocol must be reviewed and approved prior to implementation of the protocol.
- The form below must be included in the communication.
Exempt applications
- Exempt applications will not be input into the Cayuse HE system.
- Remember that if there are changes to an exempt protocol, these must be communicated by email to the IRB prior to implementation.
- If the change results in an increased risk to participants, or in the protocol no longer being exempt, a new application must be submitted.
- The IRB will notify the researcher whether the change requires a new application (in Cayuse HE).
Expedited/Full board protocols
- These protocols will be input into Cayuse HE as "Legacy" protocols.
- This includes reliance protocols, where the University of Akron is the IRB of record.
- Any changes, incidents, or closures to expedited or full board protocols on or after November 15, 2023 must be submitted in Cayuse HE.
- In order to facilitate this, researchers should contact the IRB at least 2 business days in advance (where possible).
- IRB staff will input the currently approved protocol into the system.
- After the currently approved version is in the system, the PI will be notified that they may submit the intended action.
Reliance
- Reliance protocols, where the University of Akron relies on another institution, will not be input into Cayuse HE.
- Reliance protocols, where the University of Akron IRB is the IRB of record and the protocol was approved through expedited or full board procedures, will be input into Cayuse HE.
Submissions are received by the IRB after all key personnel (PI, Co-PIs, Faculty advisor) have CERTIFIED the Submission. See the flow chart below.
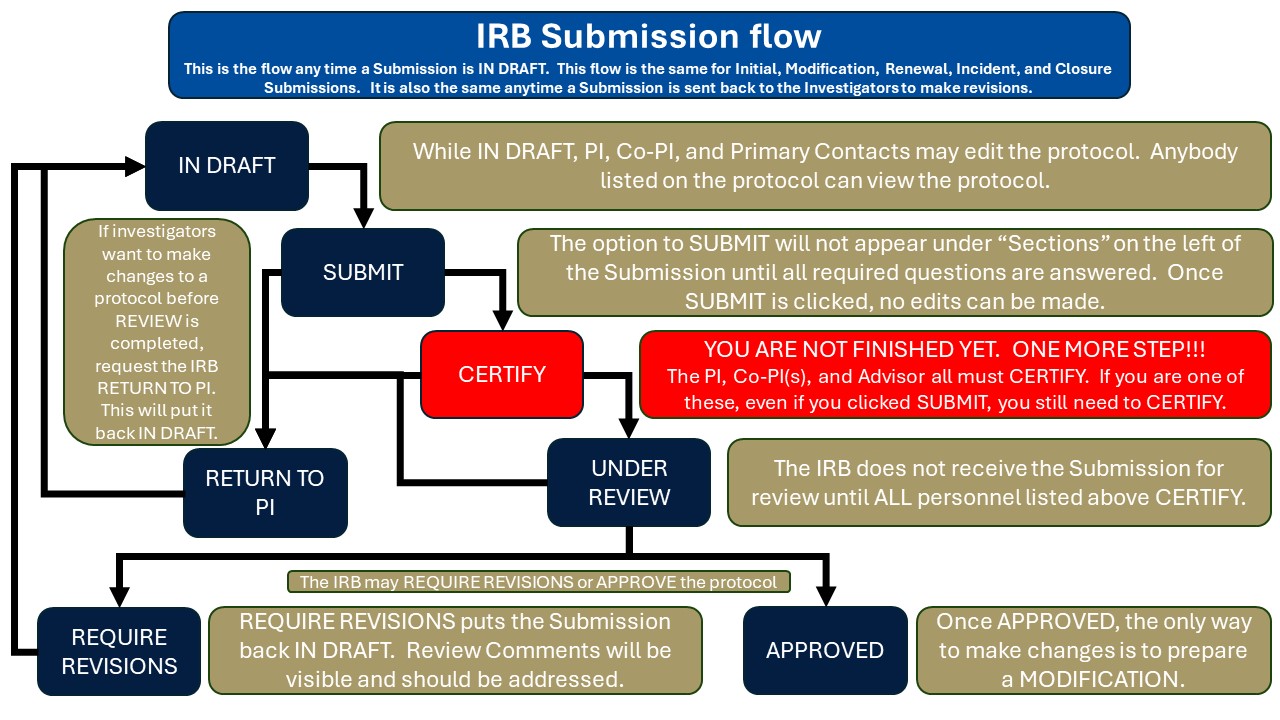
Who can view the protocol?
- All personnel listed on the protocol application will be able to view the protocol, including "Other Personnel"
- All personnel interacting with human subjects or identifiable data should be listed as some category of study personnel.
Who can edit the protocol? Note: This group of study personnel is also able to create follow-on submissions (e.g. modification, incident report, closure, etc.)
- PIs
- PC (Primary Contact)
- Co-PIs/Co-Is
- Faculty sponsors
Who receives study communications?
- PIs
- PCs
- Co-PIs/Co-Is
- Faculty sponsors
Yes and no. Because the protocol application has built in logic, there is no single application that includes all the questions a person may encounter. However, there is a protocol application pdf at this link, which provides a list of the most common types of application questions you are likely to encounter.
The following study personnel must certify the protocol before the IRB receives it for review:
- PI
- Co-PI/Co-Is
- Faculty advisors for projects where the PI is a student
Once an application is submitted, PIs and Co-Is receive notification that they must certify the submission prior to the IRB receiving the submission to review. Once the submission is in this certification workflow, no edits may be made to the submission.
In order to make edits to the submission, it must be returned to the investigators. PIs and Co-Is may return a submission to the investigators. To do this, simply navigate to the submission dashboard (within the study and submission itself) and in the upper right area of that page, there is a "Return" button. Click the button.
For a short video of how to "Return" a submission so that it may be edited by the key personnel, click here (Note: the video also discusses how to certify the protocol).
