Chemistry Research
Faculty, graduate students, and undergraduates are actively engaged in chemical research. Basic research is done in all the general areas of chemistry: analytical chemistry, biochemistry, inorganic chemistry, organic chemistry, and physical chemistry including both experimental and computational research areas. The research activity is supported by the major instrument centers in the department: Magnetic Resonance Center, Mass Spectrometry Center, Center for Laser Spectroscopy, the X-ray Crystallography Center, as well as High Performance Computing resources (ARCC HPC cluster) provided by the University. You can read about the faculty research areas by following the links below. In addition, synopses of selected graduate and undergraduate research projects are highlighted.
[Pt@Sn17]4−: An Exception from Group 14 Endohedral Clusters
Graduate Student: Chad Studvick
Mentor: Dr. Ivan Popov
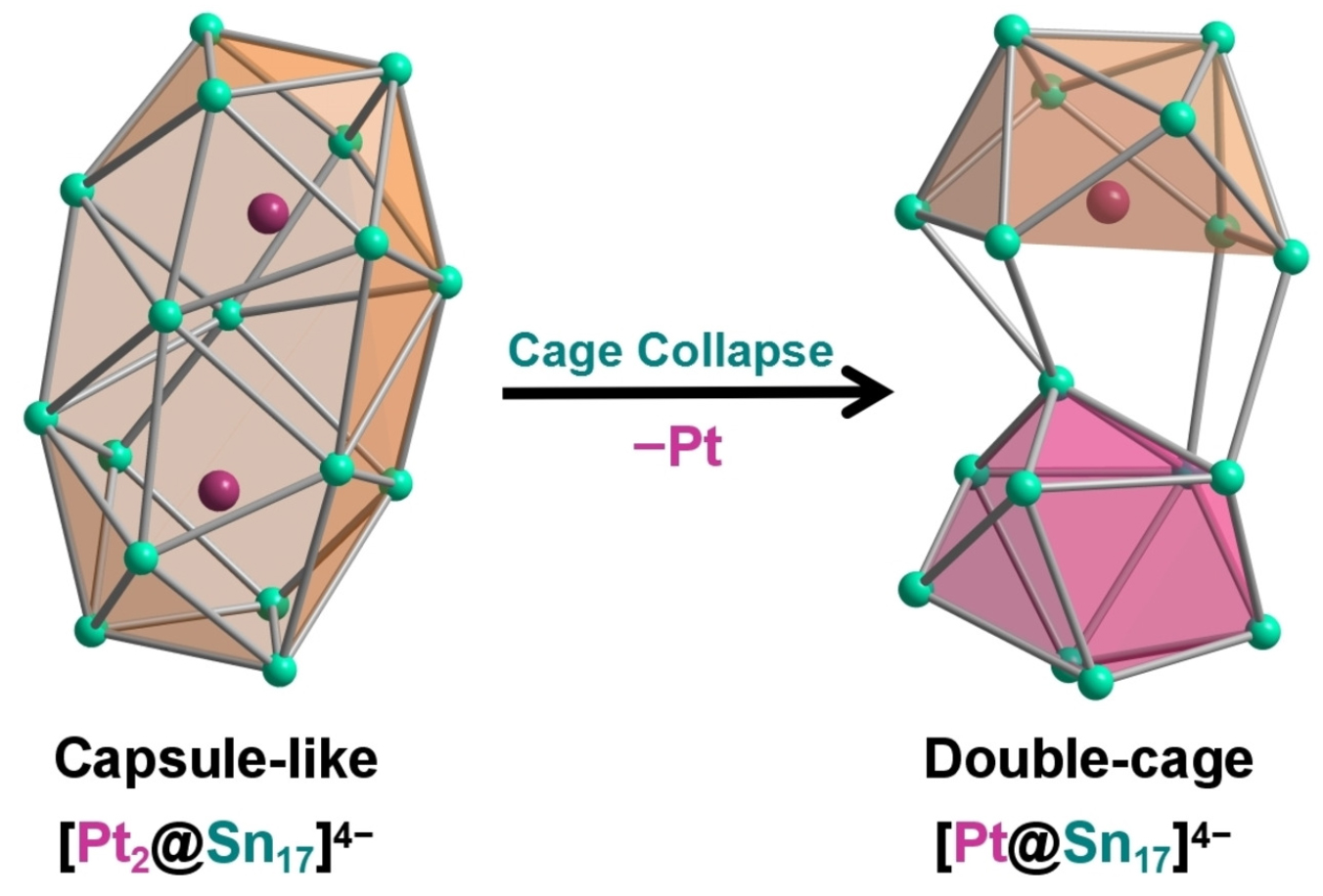
Group 14 endohedral clusters containing a metal center inside usually possess a single cage topological structure. A recently reported [Pt@Sn17]4− is found to exhibit an unexpected single-metal-filled double-cage framework, representing the largest group 14 intermetalloid cluster encapsulating a single transition metal atom. DFT calculations show that the capsule-like architecture of [Sn17]4−, similar to that found in the previously reported [Pt2@Sn17]4−, is unstable if filled with a single Pt atom and collapses to the title cluster upon geometry optimization. Deviation of the central Sn atom occurs due to the vibronic coupling as a consequence of pseudo-Jahn-Teller distortion leading to the bent Cs-symmetrical structure, in contrast to the more symmetrical D2d cage previously reported in [Ni2@Sn17]4−.
This research was published in Chemistry - A European Journal receiving the highest recognition from the reviewers and was selected as a VIP paper by the journal. In addition, it was chosen to be featured on the journal's inside cover.
Divergent Stabilities of Tetravalent Cerium, Uranium, and Neptunium Imidophosphorane Complexes
Graduate student: Chad Studvick
Mentor: Dr. Ivan A. Popov
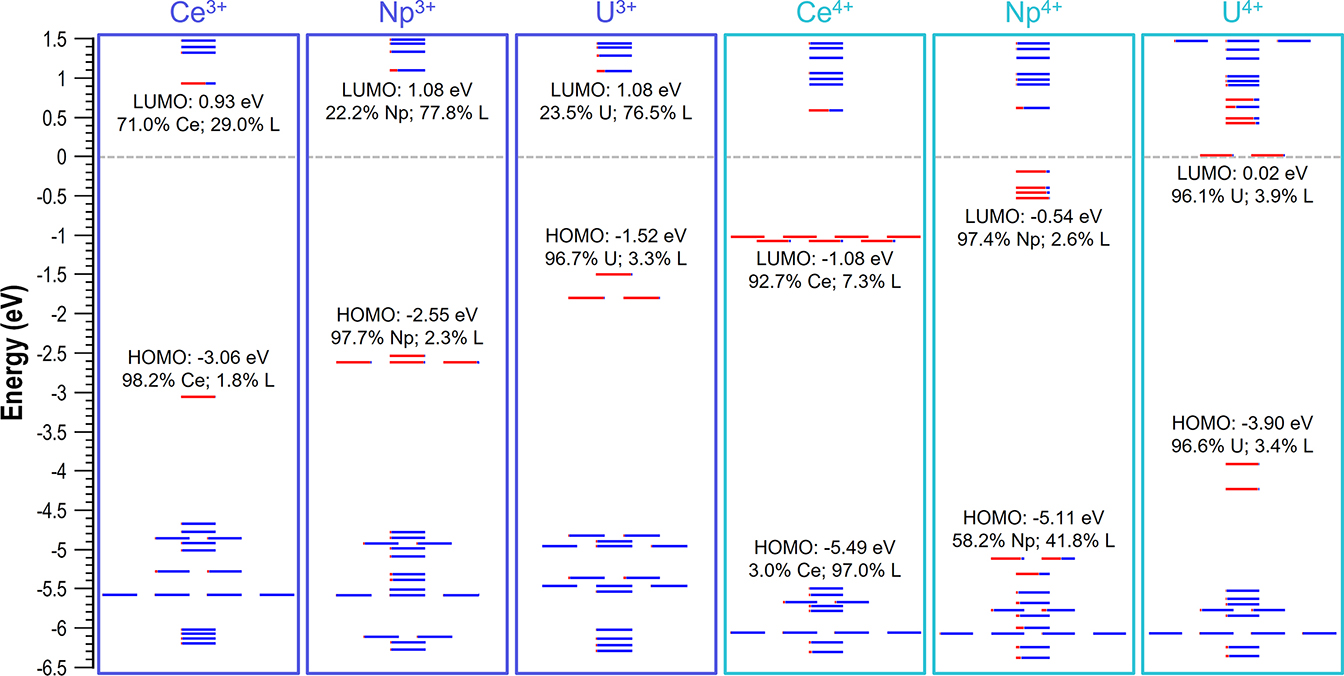
DFT studies on the experimentally synthesized imidophosphorane complexes of Ce, U, and Np show that the cathodically shifted M4+/3+ redox potentials afforded by the strongly donating NPC ligands (NPC = [N=PtBu(pyrr)2]−; pyrr = pyrrolidinyl)) can be rationalized by their relative LUMO energy values, which can therefore be instrumental in guiding the synthesis of similar f-element complexes. The metal f-dominant LUMO of the U4+ species (+0.02 eV) resides appreciably higher in energy, i.e., by +1.10 eV and +0.56 eV from that of Ce4+ and Np4+ complexes. This indicates the following order in the capacity of these species to be reduced: Ce4+>Np4+>U4+, making trivalent [U3+(NPC)4]− complexes inaccessible.
The article describing collaborative work of Prof. Popov and Prof. La-Pierre (Georgia Tech) groups is published in Angewandte Chemie International Edition.
Contact us:
Phone: 330-972-8385
Fax: 330-972-6085
Email: garciaj@uakron.edu
Mail
The University of Akron
Department of Chemistry
Akron, OH 44325-3601
Location
103 Knight Chemical Laboratory
(KNCL on map)
190 E. Buchtel Common
Akron, OH 44325-3601
