
Grades: 5-8
Author: Tess Ewart
Condiment Diver Lesson Plan
Module Description
This module's hands-on activity uses a condiment packet to teach how fish use their swim bladders to rise and descend in the water and other practical applications. Participants will discuss concepts of density, buoyancy, and sinking and floating - very difficult to teach. In addition, participants will discuss the best practices of using a model.
Several squeeze condiment packets (soy sauce, ketchup, mustard, etc.) Many fast food restaurants, such as McDonald's, Arby's, and Burger King, will donate the condiments. * Not all condiments behave the same; therefore it is recommended that they be tested beforehand. Have twice the number of condiment packets that you think you will need. No cost to the presenter.
Clear plastic bottle with tight-fitting lid (2-liter bottles - one for each participant); Wide-mouth bottles are easier to use than regular water bottles in case it is necessary to remove the packet from the bottle. Participants can be instructed to bring their own bottle - no cost to the presenter.
We recommend that the presenter build at least one "original Cartesian diver" (see below in Preparation) so that participants can observe the change of water in the diver to infer why it sinks or floats.
*Optional: a pair of tweezers to help retrieve the condiment packets from the bottle of water - when the condiment packet needs to be exchanged.
Bucket or large container to empty the bottles of water - when the condiment packet needs to be exchanged.
Water to fill the plastic bottles: Gallon milk jugs and funnels work well
Paper towels for spills and clean-up
Engagement
Open the discussion of today's session with a few pictures/video clips related to density - a submarine rising and sinking, for example. Tell participants that today they're going to make a model of the submarine using their 2-liter bottle, condiment packets, and water.
Assessment: The evaluation is informal. Monitor the participants' discussions for understanding of scale and non-scale models. Encourage all members of the group to share their use of models.
Exploration
Before beginning this activity, review safety procedures with participants; make sure that they know they are not to taste/eat anything in the science lab.


Screw the cap on tight.
Assessment: Monitor the participants' work to make sure they are following the correct procedures. Check to see that each member of the group is participating. Monitor the discussion for connections to the submarine in the opener.
Explanation
Pass around the "original Cartesian diver" while you hold a discussion covering the following:
Assessment: Have participants create a concept map for buoyant force and density. Include information that shows objects that float on the surface of water, objects that float between the surface and the bottom, and objects that sink to the bottom. Name three things that can be done to change the density of an object. (Change its shape, change its mass or change its volume.)
Elaboration
Return to the picture/video clip of the submarine and hold a discussion about similarities and differences between the submarine and the model. Is the condiment diver a good model for its target (the submarine)? Why or why not? Might its use lead to any misconceptions? See the AGPA Best Practices discussion of models for assistance. Ask participants to share other models that they use in their science teaching. Decide if these models are "sound" and not misleading. Give participants time to write a plan to use a model to teach an abstract concept to their students. Use Lesson Plan Template to write the plan.
Assessment: As time permits, give participants an opportunity to share their classroom implementation plans. If possible, follow up with participants as they carry out their plans in their classrooms. Look for evidence of the use of multiple models and questioning/explanations to students to compare the model to the target that it represents.
Density can be a difficult concept to teach; many experiences with mass, volume, and density make the concepts easier to understand and, therefore, easier to present to middle school learners. Participants will make a Cartesian diver with simple materials and use it to explore their own understanding of density, and why objects sink/float. In addition, this model gives participants a model to analyze for its use in middle school science.
NSES Teaching Standards: B
Teachers of science guide and facilitate learning. In doing this teachers
NSES Professional Development Standard A, B:
1.5 to 2 hours
The original Cartesian Diver is made with a pipette and a hex nut that can fit snugly onto the stem of the pipette. Plastic Cartesian Diver Pipettes from Educational Innovation , Inc. www.teachersource.com (2006) #pp-222A Bag of 100 $6.95 & Brass hex nuts #CD-3 Bag of 100 $9.95
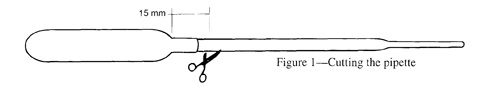
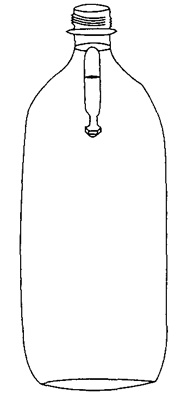
The pipette sinks to the bottom of the bottle when it is squeezed, and rises when you release the squeeze. If it doesn't work, adjust the amount of water in the pipette, by adding or subtracting water in the pipette.
Remind participants to use materials for the intended purpose; do not eat or taste anything in the science lab. Be careful about spills. Participants will want to take their diver with them for use in implementing the student lesson plan in their science classes.
Have each group of participants suggest how they have effectively used physical models in their classrooms. Please see assessment suggestions throughout the module. Visit the participants' classrooms to observe implementation of the concepts/methods discussed in today's session.
Whether an object sinks or floats is dependant on whether the object's density is greater or less than the liquid it is in.
Since density is a comparison between the mass of the object to the volume of the object, if you add to the mass without changing its volume, the density will increase. If you keep the mass the same, but decrease the volume, the density will decrease. Since gas/air can be compressed, when you apply pressure to the bottle, you also apply pressure to the air in the packet which in turn will decrease the volume of the packet, making the packet less dense.
Density = mass /volume. A pan balance is the instrument used to find the mass of an object.
Most of the sauces in the condiment packets are denser than water which should make the packets sink, but there is also some air in the packets. It is the air that helps the packets float. PictureWhen you squeeze the bottle, you put pressure on the bottle, which increases the pressure on the water, which in turn increases pressure on the packet causing the air to compress. This compression makes the packet smaller, which causes the packet to sink/dive. When you release the pressure on the bottle, the packet returns to the original volume, causing it to rise.

Submarines use the same principle to float and dive.
Scuba divers add weights/mass to keep from floating.
If the density of an object is less than the density of water, it will float. If the density of an object is greater than the density of water, it will sink.
None available for this module.
For more information about the use of models in teaching earth and space science, see: Gilbert, S. W. & Ireton, S. W. (2003). Understanding models in earth and space science. Arlington, VA: National Science Teachers Association Press.
Download Lesson Implementation Template: Word Document or PDF File
Seat everyone in groups with diversity in mind. Make sure each person participates in the discussions.
Discuss with participants the possibility of using condiment divers (already made up and functioning as intended) with students as a discrepant event (see AGPA Best Practices link for a detailed description of discrepant event). Have students try to figure out why the packet bobs up and down with the application of squeezing. Ask students to make the link between the diver and the submarine or the air bladder in fish.
Condiment Diver: www.wildfiles.tv
Eric Muller: originally published in the Physics Teacher, May 1996
AGPA version by Diane Zak