
Return to Lesson Plan Index
Printer Friendly Version
Grades: 9-12
Author: Dr. Carin A. Helfer
Source: Teegarden, D. (2004). Interfacial Step-Growth Polymerization: Synthesis of Nylon. Polymer Chemistry: Introduction to an Indispensable Science (pp. 228-230). Arlington: NSTA Press.
In this lesson, students will combine two monomers to make nylon 6,6, which is a polymer.
What should students know as a result of this lesson?
What should the students be able to do as a result of this lesson?
* This lesson requires potentially hazardous materials. Please review the Material Safety Data Sheets for the reactants and products carefully before attempting this lesson.
Engagement
Prior training in chemistry is necessary before attempting this lesson plan. The experiment can be potentially dangerous and could result in injury. The lesson must be conducted at one's own risk. Please review the Material Safety Data Sheets for the reactants and products carefully before attempting this lesson.
The students should have a basic understanding of polymers before attempting this lesson. A multimedia presentation that introduces the concept of polymers can be found at the AGPA website: http://www.agpa.uakron.edu/p16/what-are-polymers.php
As an introduction to polymerization and to engage the students, show the video below of a Polymer Science graduate student performing a polymerization in a laboratory hood.
After seeing the polymerization, tell the students that they will perform a polymerization for themselves or watch a demonstration of a polymerization in their classroom.
Assessment: Through a discussion on polymers, determine that the students have a basic understanding of polymeric materials before starting the Exploration.
Exploration
This lesson can be done as a demonstration for the whole class or with the students in small groups. The following procedure is appropriate for small groups. Scale-up as necessary for a classroom demonstration. While handling the chemicals, wear chemical-resistant gloves, safety goggles, and a laboratory apron.
Pour 5 mL of the hexamethylenediamine/sodium hydroxide solution into a small glass beaker. Carefully add 5 mL of the adipoyl chloride/hexane solution to the beaker attempting to not mix the two layers. This can be accomplished by tilting the beaker slightly and pouring the solution down the side of the beaker.
At the interface, the reaction occurs with one of the products being nylon. Use forceps to pull the nylon from the interface and wind the polymer around the stirring rod. Continue to wind the polymer onto the stirring rod. When all of the nylon (or a sufficient amount) has been collected, put the polymer into the large glass container of water to wash. Leave the polymer in the water overnight, remove it, and then allow the polymer to dry. When the nylon is odor-free, it is safe to handle without gloves.
Dispose of any leftover reagents by stirring together to allow them to fully react. Transfer any unreacted liquid into a hazardous waste container for proper disposal. The solid nylon that has been washed and dried can be disposed of in the trash.
Assessment: Monitor the students' lab work to ensure that safe lab procedures are being followed.
Explanation
Polymers are very large molecules that are made by combining smaller molecules, called monomers, together. In this experiment, a step-growth polymerization occurs at the interface of the two monomers. The monomers are each dissolved in a solvent. However, neither monomer is soluble in the solvent of the other monomer.
The reaction that occurs is as follows:
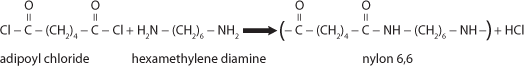
Ask the students to describe the properties of the nylon produced. Some additional questions that can be discussed are as follows:
Assessment: Monitor that the students understood the polymerization of nylon.
Elaboration
The number of natural (occurring in nature) and synthetic (man-made) polymers is extremely large. Have the students identify a polymer of interest and research this material. The instructor can decide if the students should write a report, create a presentation, or both, for the student's chosen polymer.
Assessment: Create a rubric to grade the report and/or presentation.
The instructor must have training in chemistry and chemistry lab before attempting to allow students to perform this reaction.
The students need to have basic chemistry knowledge and basic polymer knowledge.
NGSS Standards:
Common Core Standards:
National Standards:
Ohio Standards:
Step-growth polymerization was originally called condensation polymerization according to W. H. Carothers. In condensation polymerization, a small molecule is lost during the reaction. In the polymerization in this experiment, HCl is the small molecule that is formed in addition to the polymer (nylon). P. J. Flory modified the original classification of Carothers to emphasize the mechanism of the polymerization. The other type of polymerization is known as addition polymerization. In addition polymerization, the loss of a small molecule does not occur during the reaction.
The Macrogalleria website created by the Department of Polymer Science at The University of Southern Mississippi has additional details on nylons at: http://pslc.ws/macrogcss/nylon.html
Billmeyer, Jr., F. W. (1984). Textbook of Polymer Science (pp. 25-26). New York: John Wiley & Sons, Inc.
Hexamethylenediamine/sodium hydroxide solution is toxic if ingested and is corrosive.
Adipoyl chloride/hexane solution, a flammable liquid, is toxic if ingested or inhaled.
Work in a fume hood or in a well-ventilated room to avoid breathing any vapors.
Avoid skin contact by wearing a chemical-resistant apron, chemical-resistant gloves, and chemical safety goggles.
Do not handle the nylon material without chemical-resistant gloves until the polymer has been thoroughly washed.
Polymers are considered a modern material because man-made polymers have only been in existence since the mid-1800's. Today, many scientists/engineers around the world are working on creating new polymers to meet the specific needs for better physical properties. Through chemistry, structure, and processing, a wide variety of final properties can be produced.
Assessments are completed during each step of the Learning Cycle.
Grouping Suggestions: If this lesson is not done as a demonstration, it can be performed in groups of 3-4 students.
Pacing/Suggested Time: Two 40-minute class periods will be necessary for the Engagement, Exploration, and Explanation steps. The Elaboration will take additional class periods with the time depending on the Elaboration.
None